What an animal eat is key in shaping its interactions with its environment and other species. Like many fundamental ecological questions, it can be unexpectedly difficult to answer. Obviously, this question is particularly difficult for cryptic or wide-ranging animals, which are difficult to observe directly.
This is very true for marine predators such as dolphins and whales, which can travel large distances, spend most of their lives underwater and often occur in environments which are difficult and expensive to get to (not to mention the risk of getting your field notebook wet).
Killer whales, the largest of the dolphin species – are apex predators in ocean ecosystems. Their recorded prey includes more than 140 species, ranging in size from fishes weighing a few grams to baleen whales weighing tens of tons.
Marion Island, an isolated South African island in the vast Southern Ocean, is frequented by a small population of killer whales [1]. My colleagues (at the University of Pretoria’s Mammal Research Institute) and I have been studying this population for over a decade now and we know, from observing them directly, that they eat seals and penguins which breed at the island [2]. But we are very interested in what they might eat when we can’t observe them, especially when they travel hundreds of kilometers away from the island [3]. This is important because their diet determines what role they play in this particular ecosystem.
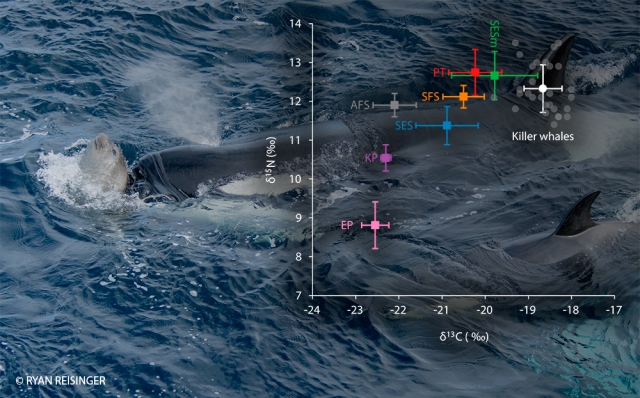
We turned to a technique called stable isotope analysis, which makes use of the fact that the proteins in an animal’s diet are incorporated into the animal’s body tissues in a predictable way. Literally, ‘you are what you eat’ [4]. The ratio of carbon stable isotopes – ẟ13C – can be used to trace the source of carbon in an animal’s tissues. In the Southern Ocean it is often used as an indicator of the latitude at which an animal has been foraging [5]. The ratio of nitrogen stable isotopes – ẟ15N – is used as an indicator of the trophic level of an animal (how high it is in the ‘food chain’). From a small piece of skin which we collected from free-ranging killer whales using a crossbow [6], we could measure both these values, and we report the results in a paper entitled “Variation in the diet of killer whales Orcinus orca at Marion Island, Southern Ocean” in the journal Marine Ecology Progress Series [7].
We found, unsurprisingly, that killer whales at Marion Island are indeed apex predators. But the ẟ15N values weren’t quite as high as we expected, which suggests that the killer whales are also eating prey of a lower trophic level than just seals and penguins, perhaps fishes and squids. Also, variations in ẟ13C values suggest that killer whales forage slightly north of Marion Island when they are not patrolling the island’s waters for seal and penguin prey. When we compared these values among the different social units [8], we didn’t find any substantial differences.
Stable isotope analysis is not a silver bullet – it has some shortcomings including the fact that an animal’s specific diet usually can’t be identified. But in this case, it has given us some insight into the unseen ecology of killer whales in a remote area, and it has brought up some interesting questions which need answering.
References
- Reisinger RR, de Bruyn PJN, Bester MN (2011) Abundance estimates of killer whales at subantarctic Marion Island. Aquat Biol 12:177–185 http://dx.doi.org/10.3354/ab00340
- Reisinger RR, de Bruyn PJN, Tosh CA, Oosthuizen WC, Mufanadzo NT, Bester MN (2011) Prey and seasonal abundance of killer whales at sub-Antarctic Marion Island. African J Mar Sci 33:99–105 http://dx.doi.org/10.2989/1814232X.2011.572356
- Reisinger RR, Keith M, Andrews RD, de Bruyn PJN (2015) Movement and diving of killer whales (Orcinus orca) at a Southern Ocean archipelago. J Exp Mar Bio Ecol 473:90–102 http://dx.doi.org/10.1016/j.jembe.2015.08.008
- DeNiro MJ, Epstein S (1976) You are what you eat (plus a few per mil): the carbon isotope cycle in food chains. GSA Abstracts with Programs 8:834−835
- Jaeger A, Lecomte VJ, Weimerskirch H, Richard P, Cherel Y (2010) Seabird satellite tracking validates the use of latitudinal isoscapes to depict predators’ foraging areas in the Southern Ocean. Rapid Commun Mass Spectrom 24:3456–60 http://dx.doi.org/10.1002/rcm.4792
- Reisinger RR, Oosthuizen WC, Péron G, Cory Toussaint D, Andrews RD, Bruyn PJN de (2014) Satellite Tagging and Biopsy Sampling of Killer Whales at Subantarctic Marion Island: Effectiveness, Immediate Reactions and Long-Term Responses. PLoS One 9:e111835 http://dx.doi.org/10.1371/journal.pone.0111835
- Reisinger RR, Gröcke D, Lübcker N, McClymont E, Hoelzel AR, de Bruyn PJN (2016) Variation in the diet of killer whales Orcinus orca at Marion Island, Southern Ocean. Mar Ecol Prog Ser 549:263–274 http://dx.doi.org/10.3354/meps11676
- Reisinger RR, Janse van Rensburg C, Hoelzel AR, de Bruyn PJN (in press) Kinship and association in a highly social apex predator population, killer whales at Marion Island. Behavioral Ecology http://dx.doi.org/10.1093/beheco/arx034